
Density functional theory calculations reveal that Pd atom forms covalent-like bonding with adjacent P atoms, wherein H atoms tend to adsorb, aiding the dissociative adsorption of H2. Metallic Pd1/BP SAC shows a highly selective semi-hydrogenation of phenylacetylene toward styrene, distinct from metallic Pd nanoparticles that facilitate the formation of fully hydrogenated products. Unlike other 2D materials, BP with relatively low electronegativity and buckled structure favors the strong confinement of robust zero-valent palladium SACs in the vacancy site. Here, it is demonstrated that 2D black phosphorus (BP) acts as giant phosphorus (P) ligand to confine a high density of single atoms (e.g., Pd1, Pt1) via atomic layer deposition. However, single atoms often bond to the support with polarized electron density and thus exhibit a high valence state, limiting their catalytic scopes in many chemical transformations. Single-atom catalysts (SACs) represent a new frontier in heterogeneous catalysis due to their remarkable catalytic properties and maximized atomic utilization. Thus, this work establishes K2V3O8 nanorods as a novel material for photocatalysis and also explores their structure–property relationships for achieving optimal photocatalytic activity. The tuned nanorods demonstrated excellent photocatalytic activity, achieving 93% degradation of complex pharmaceutical levofloxacin and a robust photocurrent density of 0.7 mA/cm2 at 1.23 V vs RHE. The spectroscopic analyses have been theoretically studied by generating electronic structures via DFT calculations. Various structural and optoelectronic analyses have been performed to establish the enhanced charge migration and charge separation efficiency along the smooth nanorod surfaces.

Morphological analyses have revealed a profound effect of SDS in tailoring the morphology of the nanorods, due to a systematic layer by layer assembly of K2V3O8 nuclei along the surfactant chains. K2V3O8 nanorods have been synthesized by a simple surfactant-assisted hydrothermal method, with the surfactant SDS being incorporated to tune the morphology of the nanorods.
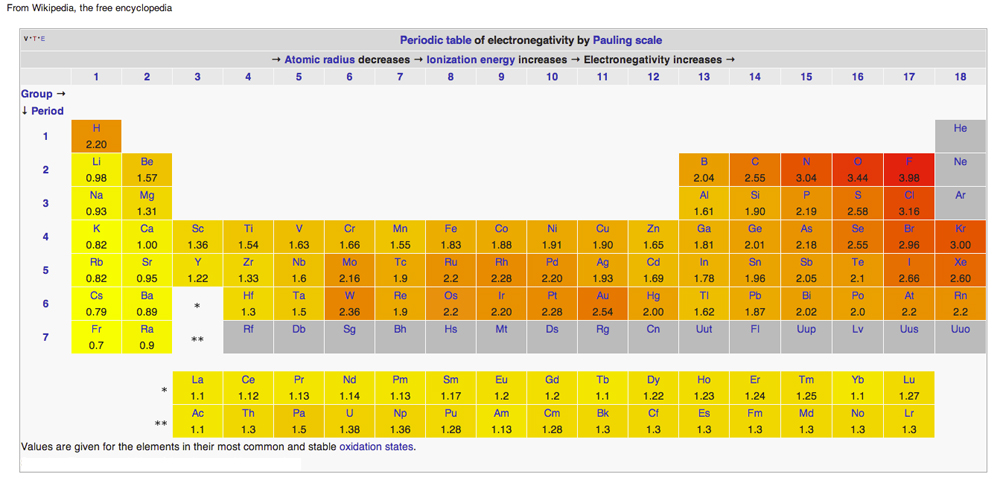
This work presents a deep investigation into the structural and optoelectronic properties of K2V3O8 nanorods for testing their photocatalytic potentials. These observations reveal the selectivity of deposition by surface functional groups. In addition, the precursors were observed to be more exothermic and show higher reaction rate constant when adsorbed on the –OH–terminated surface than on the –NH2–terminated surface. The exothermicity of the dissociative adsorption was found to be in the order of: TiI4 > TiBr4 > TiCl4. Sequential dissociation reaction mechanisms of halide ligands were systematically investigated. We consider the most favorable adsorption site in the reaction between the precursor and functional group of the surface, based on the thermodynamics and kinetics of the reaction. Herein, we present a density functional theory (DFT) study of the initial nucleation process of some titanium halide precursors (TiCl4, TiBr4, and TiI4) on Si surfaces having –OH or –NH2 functional groups. To improve the uniformity and conformity of thin films grown via ALD, fundamental understanding of the precursor–substrate surface reactions is required.
Various processes based on atomic layer deposition (ALD) have been reported for growing Ti-based thin films such as TiN and TiO2. Our work provides a new route towards the synthesis of zero-valent SACs on BP for a wide range of organic transformations. Zero-valent Pd in the confined space favors a larger energy gain for the synthesis of partially-hydrogenated product over the fully-hydrogenated one. Our DFT calculations reveal that Pd atom forms covalent-like bonding with adjacent P atoms, wherein H atoms tend to adsorb over electron-rich region for the subsequent hydrogenation. Metallic Pd1/P SAC shows a highly selective semi-hydrogenation of phenylacetylene towards styrene, outperforming high-valence Pt1/P SAC, and also distinct from metallic Pd nanoparticles that facilitate the formation of fully hydrogenated products. Here, we demonstrated that two-dimensional (2D) black phosphorus (BP) act as giant phosphorus (P) ligand to confine a high density of single atoms (eg, Pd1, Pt1) via atomic layer deposition.


 0 kommentar(er)
0 kommentar(er)
